Transcatheter heart valve implantation is a method with minimally invasive method, which helps in changing the heart valve with a prosthetic valve for the action of mitral regurgitation and severe aortic stenosis. It is an effective substitute to cumbersome open heart surgery, particularly for high-risk patients. It is a new technology that has less mortality rate and varieties of benefits over traditional surgical techniques.
The method helps to decrease severe degenerative aortic stenosis and increases the survival rate of the patients. Aged population is at a higher risk of valvular heart disease due to growing occurrence of asymptomatic severe mitral regurgitation and severe aortic stenosis. This is leading to increasing percutaneous transcatheter prosthetic valve implantation across the globe.
Market Performance of TAVI
As per the report by Grand View Research, Inc., the transcatheter heart valve market is poised to rise to a valuation of USD 8.62 billion by 2024, with the transcatheter aortic valve application segment occupying the major market share. Increasing prevalence of chronic heart diseases, increasing healthcare expenditure, and growing number of people with severe aortic stenosis are driving the growth of the market.
Furthermore, technological advancements relating to the growth of the tricuspid valve intervention, neochord systems, and implementation of harpoon are also amongst the major factors that are anticipated to propel the use of transcatheter aortic valve market over the forecast period.
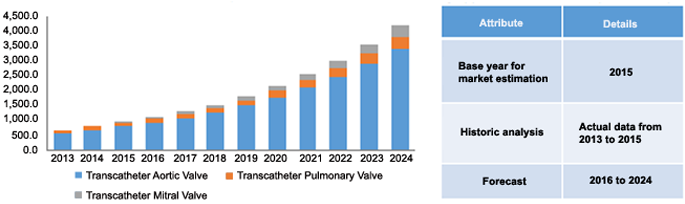
Source: Grand View Research
- Current Status of Transcatheter Heart Valve
Clinical experience with a transcatheter heart valve is dominated by the Medtronic CoreValve systems and Edwards Sapien but numerous innovative valves are challenging the market and are expanding on repositionability, design, price, and retrievability. Enhanced valve design and sizing, and smaller delivery catheters have significantly reduced post-procedural and periventricular leukomalacia (PVL) complications.
- Predicting the future of Transcatheter Heart Valve
The pharmaceutical and device industry are devising plans and proposal policies in association with the regulatory bodies to distribute transcatheter heart valve in developing countries such as India, in order to meet the huge need and restricted resources. Industries are paying attention to bringing about awareness among the physicians and people about the technology along with training of physicians, research and development of novel valves, etc.
Transcatheter heart valve is developing vastly on account of increasing funding in research and development by the significant market companies such as Medtronic, Edwards Life Sciences, Boston Scientific, and St. Jude. The technologically progressive products such as the TRINITY Valve and SAPIEN 3 transcatheter heart valve are designed to decrease the surgical problems and improve patient compliance. For example, the TRINITY TAVI system allows repositioning of the device and easy retrieval without compromising on patient treatment and safety outcomes.
- Advances and future directions of Transcatheter Heart Valve
Transcatheter heart valve offers the advantages of being minimally invasive than conventional surgical solutions and has come out as a therapeutic choice for patients with high clinical risk. However, even with its potential to lower the risk in the frail population, it originates with its exclusive set of complications. In addition, technological advancements in valve function, delivery, and structure are still remaining to try to lessen the risks. The current technological developments in transcatheter aortic valve replacement technology is showing a path to the future in this novel field.
- Challenges in the near future
Less valve durability and risk of patients during the valve change along with the risk of bicuspid aortic valve disease is a major risk in the treatment. The cerebrovascular stroke is one of the most complicated challenges for the heart valve replacement technique.
Furthermore, cost of the surgery has been a foremost concern for healthcare stakeholders and hospital administrators. The cost of transcatheter valves cost 6 times more than surgical prostheses along with the chances of dispositioning in the implantation are anticipated to hinder the growth of the transcatheter heart valve market in the near future.
Conclusion:
The expansion of the transcatheter heart valve market has been dependent on strong clinical evidence derived from randomized controlled trials and large-scale national and international offices. In the past, transcatheter heart valve has progressed into an effective and safe method with reproducible and predictable outcomes. In addition, with rising aged populace across the globe, transcatheter heart valve is likely to play a significant role in the future of cardiovascular health of individuals.