See Report #S192, “Worldwide Surgical Sealants, Glues, and Wound Closure Markets, 2013-2018”.
See Report #S192, “Worldwide Surgical Sealants, Glues, and Wound Closure Markets, 2013-2018”.
Sutures have been in use for potentially thousands of years, and staples for the last several decades. Both have frequently been the target of new development in wound closure and management, with competition in the form of advanced wound closure, whether surgical sealants, glues, hemostats, and even other mechanical wound closure. Novel wound closure technologies have decidedly gained enough credibility in clinical practice to displace volume in sutures and staples.
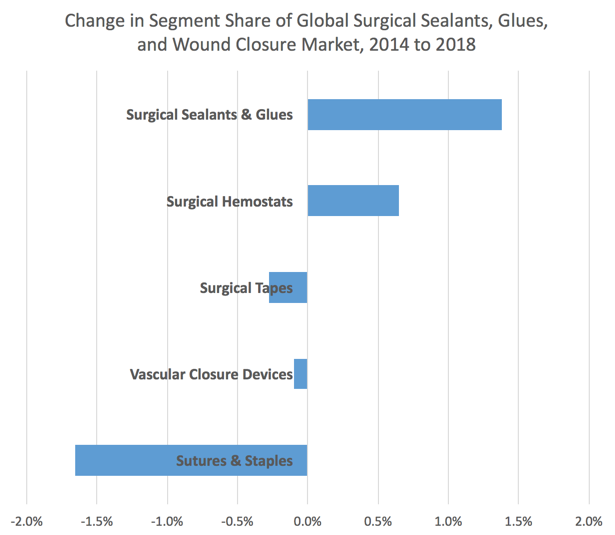
Sutures and Staples Are Not Fading…
Manufacturers of sutures and staples have not sat idly and watched their share erode. Indeed, the development of bioresorbable sutures and other novel suture types, the development of sophisticated stapling and suturing endoscopic instrumentation and other developments have begun to erode the share loss. Consequently, the shift “away” from sutures and staples has ebbed, such that the aggregate swing in market shares is no more than 3% compared to the swing projected three years ago of nearly 7% (see link).
Sutures and Staples in Wound Closure (excerpt from Report #S192)
The vast majority of sutures, staples, and endostaples are used to close procedures involving acute surgical wounds. Typically, chronic wounds do not involve the use of sutures and staple products unless some degree of surgical intervention is employed to remove necrotic tissue or to create a new acute wound bed to aid healing.
Sutures are classified as absorbable or non-absorbable; monofilament, multifilament or braided; and natural or synthetic. Absorbable or non-absorbable describes the suture’s effective life within tissue. Absorbable sutures lose the majority of their tensile strength within 60 days after use. Non-absorbable sutures are resistant to living tissue and do not break down. Monofilament, multifilament, and braided describe the structure or configuration of the suture based on the number of strands used to manufacture the product. Natural or synthetic refers to the origin of the suture. Natural suture materials include surgical gut, chromic gut, catgut and silk. Catgut is made from the natural collagen fibers found in the intestine of sheep, goats, cattle, hogs and horses. (It was never made from the gut of cats.) It is debatable whether catgut should continue to be used for suturing wounds, since cotton is cheaper and cotton or synthetic threads are less likely to cause infection. Synthetic suture materials include nylon, polyester, stainless steel, polypropylene, polyglycolic acid (PGA), polyglycolide-co-caprolactone (PGCL), and polydioxanone.
Suture products consist of two component parts, the needle and the suture. These can be found in a wide range of sizes and types, made of a range of materials, and the method of attachment of the suture to the needle can involve a variety of methods. Sutures are divided into braided and monofilament categories. Braided sutures are typically more pliable than monofilament and exhibit better knot security. Monofilament sutures are wirier and may require a more secure knot; however, they cause less tissue drag than braided sutures, a characteristic that is especially important in cardiovascular, ophthalmic and neurological surgery
Stapler devices are an evolution of suture technology. The goal of stapler products is to avoid infection and make the wound closure procedure easier and faster. Staples are made of stainless steel and biomaterials and are used to join internal tissues, reconstruct or seal off organs, remove diseased tissue, occlude blood vessels, and close skin incisions and lacerations. They are primarily used during surgery as internal and/or external closure devices.
Staples are available in an assortment of sizes and features and stapler devices have been developed for specific procedures as well as for multiple uses.
Internal staplers are used to approximate (or close) internal tissues and organs. The devices may be reusable or disposable. Some disposable staplers may be reloaded several times during the course of a single patient surgical procedure, before being discarded.
The most recent internal staplers are used to perform minimally invasive surgical procedures. These allow the surgeon to endoscopically secure internal wounds instead of having to operate through an open procedure. Moreover, internal biodegradable staples obviate the need for staple removal. Such staples are ideally suited to laparoscopic surgery and are delivered via procedure-specific laparoscopic instruments. However, most staples are still made of stainless steel and when used for internal stapling procedures, whether open or laparoscopic, are not removed after healing. Skin staples are removed after the incision is healed.
Probably the major benefit of staples is that they can be applied more rapidly than sutures and can be placed precisely without requiring the skill necessary for suturing. This also means increased safety for the patient, and patients can often be discharged more rapidly if procedures are stapled rather than sutured.
While cosmetically acceptable results are usually obtained, staplers normally are not used in highly visible areas such as the face. Here, surgeons will still close by hand to minimize any scarring. In many skin closure procedures, sutures have begun to be replaced by cyanoacrylate glues. However, the ideal alternative to suturing has not yet been developed; for example, cyanoacrylate glues used for external skin closure are only one-fifth as strong as sutures.