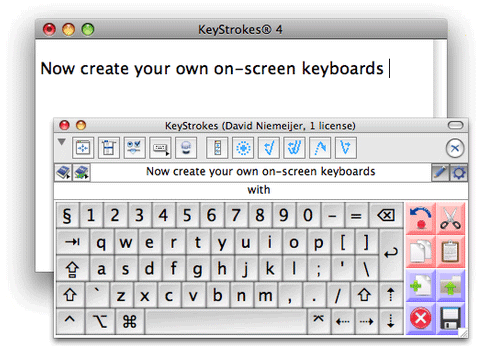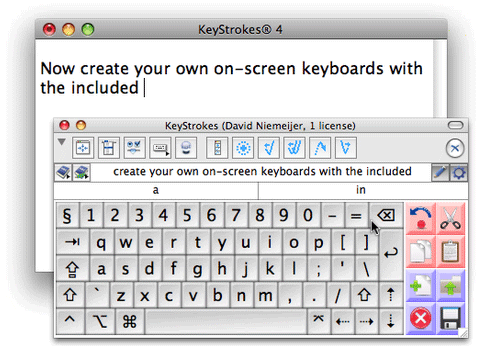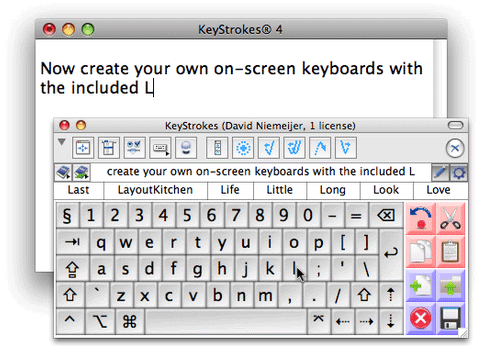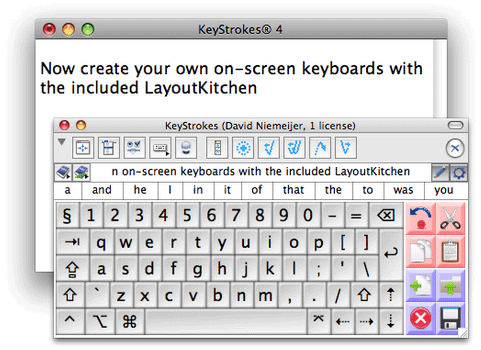
The drug is known as a rapid antipsychotic treatment and was turned down in 2010 as the FDA was concerned about the side effects of bronchial spasms.
turned down in 2010 as the FDA was concerned about the side effects of bronchial spasms.
From the website:
turned down in 2010 as the FDA was concerned about the side effects of bronchial spasms.
From the website:
“All of our product candidates are based on our proprietary technology, the Staccato® system. The Staccato system vaporizes excipient-free drugs to form a condensation aerosol that, when inhaled, allows for rapid systemic drug delivery. Because of the ideal particle size of the aerosol, the pure drug is quickly absorbed through the deep lung into the bloodstream,  providing speed of therapeutic onset that is comparable to intravenous (IV) administration but with greater ease, patient comfort and convenience.
providing speed of therapeutic onset that is comparable to intravenous (IV) administration but with greater ease, patient comfort and convenience.
Alexza’s lead program is ADASUVE™ (Staccato loxapine or AZ-004), being developed for the acute treatment of agitation in patients with schizophrenia or bipolar disease, and has regulatory processes ongoing in both the United States and Europe”.
They also have other inhalable drugs in the pipeline to include migraine headache relief, insomnia and pain relief. You can see at the link below another company has their spray inhalable system already FDA approved. BD
Nasal Spray Drug Sprix Gets FDA Approval – Inhale and Sniff For Pain Relief
Alexza Pharmaceuticals Inc. (ALXA), the company that hired Lazard Ltd. (LAZ) a year ago to explore strategic options including selling itself, won U.S. approval of its rapid antipsychotic medicine.
The Food and Drug Administration cleared the inhaled treatment Adasuve for agitation associated with schizophrenia or bipolar disorder in adults, the company said today in a statement. The drug, recommended by European regulators for approval Dec. 14, will be the Mountain View, California-based company’s first product on the market.
The FDA also required Alexza to conduct a large post- marketing clinical trial of patients to assess “the real-world use” of the drug, the company said.
Alexza in February fired 29 employees, or 38 percent of its workforce, to focus on development of Adasuve and sold 44 million shares, raising about $20.4 million, the company said in a statement. The company had hired Lazard in December 2011 to explore whether it should find a buyer.