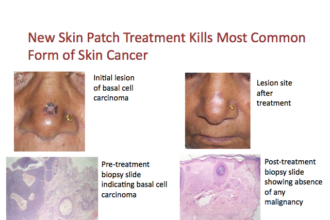
Monica Healthcare (based in the UK), has announced that the US Food and Drug Administration (FDA) has granted regulatory clearance of their Monica AN24 wireless fetal monitor for use during Labor and Delivery. This FDA clearance enables Monica technology to be utilized for intrapartum term monitoring for all singleton births in the USA. The Monica AN24 uses wireless, non-invasive technology to collect real-time electrical signals from the abdomen of a pregnant mother. This method of using electrophysiological signals differs from current external monitoring devices that collect FHR and uterine activity data based on physical changes (e.g. change in reflected sound waves and changes on strain gauge) that may cause problems in data interpretation such as discerning FHR vs. MHR data. The Monica AN24 does not require constant re-positioning of transducers, which is required with the current technology, especially during an epidural when the patient is on her side. Clinical trials in the US also have demonstrated that Monica performs well in obese women. Obesity in pregnancy is becoming more prevalent and acquiring accurate FHR and uterine activity signals in obese patients using conventional external monitoring is sometimes challenging. In the hospital, the Monica AN24 will be available exclusively in the USA through Glenveigh Medical. It was formally introduced earlier this year at the 31st meeting of the Society of Maternal-Fetal Medicine in San Francisco, February 9-11. The U.S. fetal and neonatal monitoring equipment markets have been exhibiting sedentary growth rates due to maturity of the markets. This saturation was caused by several factors including, the almost flat birth rate since the late 1990s and the shifting of existing monitoring equipment to the decline phase of the product life cycle due to lack of new products. The use of fetal and neonatal monitoring and diagnostics is growing in the European Union due to a combination of higher awareness among expectant mothers about the advantages of sophisticated monitoring, as well as the non-invasiveness and greater safety offered by new prenatal diagnostic techniques, according to an analysis from market research firm Frost & Sullivan. The European market for fetal and neonatal monitoring equipment earned revenues of $130.9 million in 2008, and Frost & Sullivan estimated the market to hit $161.2 by 2012. Neoventa Medical, Cervilenz, and Nascor are also making advancements in fetal care and labor devices.