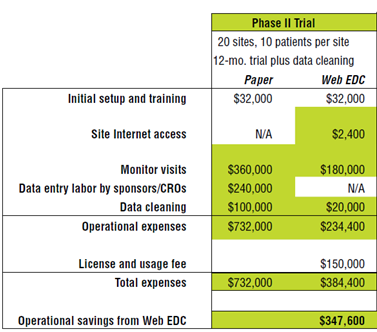
A recent publication suggests that Electronic Data Capture systems, or EDCs, should be implemented in clinical trials. The EDC software can reduce the overall trial costs by an estimated $347,600 in a phase ll trial of 20 sites, with 10 patients per site and a duration of 12 months plus data cleaning. See the table below:
In this example, monitor visits still cost $180,000. Vitaphone CTS provides solutions to make subjects monitor their vital signs at home. This can also enhance their trial compliance. Re-activity is a top priority to create better and more efficient clinical trials. The tools developed by Vitaphone CTS will send biosensor data immediately and thus ensure almost real-time data. Our medication adherence support system (MASS) stimulates the study subjects to take their study medication.
Remote monitoring and support enables pharmaceutical, biotechnology and medical device industries to focus on the development of the best drug or device.
Using telemonitoring solutions, investigators can improve patient safety. This might be achieved through faster notification of adverse events to the Principal Investigator, CRO or Sponsor. This will support earlier and better decision-making. Embrace technology.
Source: Clinovo