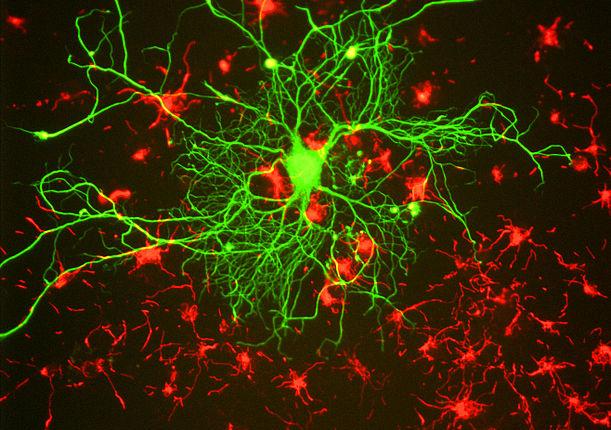
The idea behind ‘personalized’ medicine is to target the precise mutation in a patient’s genes that causes a disease. In a series of new experiments conducted at Johns Hopkins University, scientists have taken the first step toward developing personalized drugs that take direct aim at ALS and one form of dementia.
“Efforts to treat neurodegenerative diseases have the highest failure rate for all clinical trials,” Jeffrey D. Rothstein, M.D., Ph.D., a professor of neurology and neuroscience and lead author of the new research, noted in a press release. And for this reason his work is very valuable: it may pave the way to developing new treatments for two neurodegenerative diseases and so become the exception to this rule.
Repeating Genes
Amyotrophic lateral sclerosis (ALS) is often referred to as ‘Lou Gehrig’s disease’ in honor of the Yankee baseball player who died from it. This progressive neurological disease attacks the nerve cells responsible for controlling voluntary muscle movement. As cells in the brain and spinal cord waste away and die, they no longer send messages to the muscles, which begin to twitch as they weaken. Eventually, a patient is unable to move their arms, legs, and body.
Onset of the disease is typically around the age of 50 and once it begins, progress is swift: death often occurs within three to five years of diagnosis. There is no cure for ALS, which affects 20,000-30,000 people in the US, yet there is only one FDA-approved drug treatment. At best, Rothstein explained, the drug has just a small effect in slowing down the disease, which is hereditary in about 10 percent of cases. For years, researchers have been working toward new treatments for the disease.
Then in 2011, scientists discovered that more than 40 percent of patients with an inherited form of ALS — as well as 10 percent of patients with the non-inherited form — have a mutation in the C9ORF72 gene. The job of this gene is to provide instructions for making a particular protein found in many tissues. In people who don’t suffer from ALS, there are up to 30 repeats of a series of six DNA letters on the C9ORF72 gene. In people with ALS, though, this same string of DNA letters may be repeated thousands of times. The very same mutation often occurs in people with frontotemporal dementia, the second-most-common form of dementia after Alzheimer’s disease.
How did Rothstein and his team make the leap from a single gene mutation to their own discovery?
Stem Cells
The team based their experimental design on a type of stem cell known as induced pluripotent stem cells (iPS cells). These cells are adult cells that researchers have ‘genetically reprogrammed’ to act like an embryonic stem cell. Essentially, the iPS cells are forced to express genes and other characteristics of an embryonic stem cell.
To examine the C9ORF72 gene mutation, Rothstein and his team used neurons derived from the skin of people with ALS to create an entire bank of iPS cell liens. Next, Rothstein searched his bank of iPS cell lines to identify those with the C9ORF72 mutation. Then, he and his team began a series of experiments to understand exactly out how the repeating string of six DNA letters caused the death of brain cells.
What did they discover? In the iPS neurons with the genetic mutation, the RNA made from the repeating strings of DNA letters was bunching up; by producing too much of one kind of RNA and not enough of another, the C9ORF72 mutation gummed up the works. To counter this effect, the researchers developed a number of chemical compounds targeting the problem; the compounds ‘matched up’ to the repeating six DNA letters and so inhibited them, which in turn allowed the RNA to function as it should.
Future Steps
Having analyzed brain tissue from people with the C9ORF72 mutation who died of ALS, the researchers saw evidence of the same bunching up they had seen in the cultures created in their laboratory. They also discovered the same genes altered in the iPS cells as a consequence of the genetic mutation had also become abnormal in the brain tissue. In other words, what they observed in the actual brain tissue seemed to substantiate their lab work. Read here to know more about it.
“An iPS cell line can be used effectively and rapidly to understand disease mechanisms,” Rothstein noted. “Now we need to see if our findings translate into a valuable treatment for humans.”
Isis Pharmaceuticals, which helped develop many of the compounds used in the experiments, will be working with the Johns Hopkins team, Rothstein noted, and could begin testing compounds in human subjects — and not just laboratory cultures — within the next few years. In fact, plans are already underway to begin identifying a group of patients with the C9ORF72 mutation for future research.
Personalized medicine is a new treatment avenue that appeared only after scientists completed mapping the human genome in 2003. Soon, it may become a path familiar to many, including patients with ALS and dementia.
Source: Rothstein JD, Donnelly CJ, Zhang PW, et al. RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron. 2013.